PhD students Alex Niebergall and Weiyi Tang participated in a NASA-funded research cruise in the Northern Pacific Ocean as part of the Export Processes in the Ocean from Remote Sensing (EXPORTS) project. EXPORTS is a multi-institutional research initiative that seeks to develop a predictive understanding of the export and fate of global ocean net primary production and its implications for present and future climates. The students’ research project specifically looked at the role marine microbes play in drawing carbon dioxide out of the atmosphere.
Category: Lab News (Page 3 of 4)
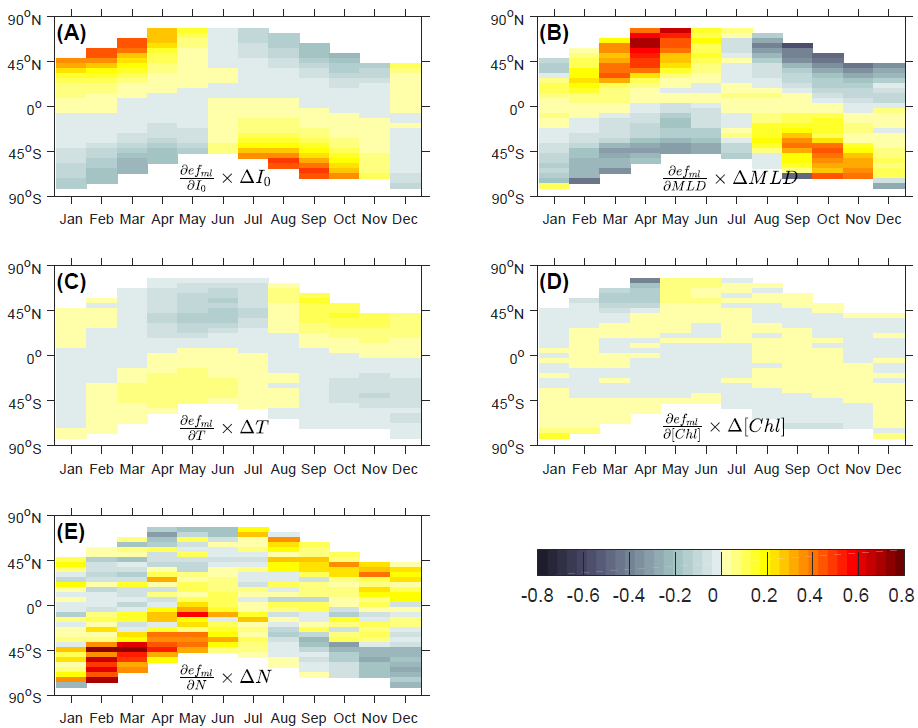
The fraction of primary production exported out of the surface ocean, known as the export ratio ( ef-ratio), is often used to assess how various factors, including temperature, primary production, phytoplankton size and community structure, affect the export efficiency of an ecosystem. To investigate possible causes for reported discrepancies in the dominant factors influencing the export efficiency, we develop a metabolism-based mechanistic model of the ef-ratio. Consistent with earlier studies, we find based on theoretical considerations that the ef-ratio is a negative function of temperature. We show that the -ratio depends on the optical depth, defined as the physical depth times the light attenuation coefficient. As a result, varying light attenuation may confound the interpretation of ef-ratio when measured at a fixed depth (e.g., 100 m) or at the base of the mixed layer. Finally, we decompose the contribution of individual factors on the seasonality of the ef-ratio. Our results show that at high latitudes, the ef-ratio at the base of mixed layer is strongly influenced by mixed layer depth and surface irradiation on seasonal timescales. Future studies should report the ef-ratio at the base of euphotic layer, or account for the effect of varying light attenuation if measured at a different depth. Overall, our modeling study highlights the large number of factors confounding the interpretation of field observations of the ef-ratio.

Hot off the press paper by Seaver Wang in The ISME Journal
Marine net community production (NCP) tracks uptake of carbon by plankton communities and its potential transport to depth. Relationships between marine microbial community composition and NCP currently remain unclear despite their importance for assessing how different taxa impact carbon export. We conducted 16 and 18S rRNA gene (rDNA) sequencing on samples collected across the Western North Atlantic in parallel with high-resolution O2/Ar-derived NCP measurements. Using an internal standard technique to estimate in-situ prokaryotic and eukaryotic rDNA abundances per liter, we employed statistical approaches to relate patterns of microbial diversity to NCP. Taxonomic abundances calculated using internal standards provided valuable context to traditional relative abundance metrics. A bloom in the Mid-Atlantic Bight featured high eukaryote abundances with low eukaryotic diversity and was associated with the harmful algal bloom-forming Aureococcus anophagefferens, phagotrophic algae, heterotrophic flagellates, and particle-associated bacteria. These results show that coastal Aureococcus blooms host a distinct community associated with regionally significant peaks in NCP. Meanwhile, weak relationships between taxonomy and NCP in less-productive waters suggest that productivity across much of this region is not linked to specific microplankton taxa.

FARACAS Concept
Because of the difficulty in resolving the large variability of N2 fixation with current methods which rely on discrete sampling, the development of new methods for high resolution measurements is highly desirable. We present a new method for high-frequency measurements of aquatic N2 fixation by continuous flow-through incubations and spectral monitoring of the acetylene (C2H2, a substrate analog for N2) reduction to ethylene (C2H4). In this method, named “Flow-through incubation Acetylene Reduction Assays by Cavity ring-down laser Absorption Spectroscopy” (FARACAS), dissolved C2H2 is continuously admixed with seawater upstream of a continuous-flow stirred-tank reactor (CFSR) in which C2H2 reduction takes place. Downstream of the flow-through incubator, the C2H4 gas is stripped using a bubble column contactor and circulated with a diaphragm pump into a wavelength-scanned Cavity Ring Down laser absorption Spectrometer (CRDS). Our method provides high-resolution and precise mapping of aquatic N2 fixation, its diel cycle, and its response to environmental gradients, and can be adapted to measure other biological processes. The short-duration of the flow-through incubations without preconcentration of cells minimizes potential artefacts such as bottle containment effects while providing near real-time estimates for adaptive sampling. We expect that our new method will improve the characterization of the biogeography and kinetics of aquatic N2 fixation rates. See Cassar et al. 2018.
 EXPORTS is a NASA large-scale field campaign that will provide critical information for quantifying the export and fate of upper ocean net primary production (NPP) from satellite observations. The overarching goal of EXPORTS is to develop a predictive understanding of the export and fate of global ocean primary production and its implications for the Earth’s carbon cycle in present and future climates.
EXPORTS is a NASA large-scale field campaign that will provide critical information for quantifying the export and fate of upper ocean net primary production (NPP) from satellite observations. The overarching goal of EXPORTS is to develop a predictive understanding of the export and fate of global ocean primary production and its implications for the Earth’s carbon cycle in present and future climates.

Lin, Y., Cassar, N., Marchetti, A., Ducklow, H., Li, Z. Specific eukaryotic plankton are good predictors of net community production in the Southern Ocean. Nature Scientific Reports, doi:10.1038/s41598-017-14109-1.

Cassar begins LabexMer Chair International d’Excellence at Université de Bretagne Occidentale






You must be logged in to post a comment.