
By Jeff Farner Budarz
As ToxInsider mentioned at the beginning of November, nanoparticles have generated a lot of attention and interest – both as technological game changers and harbingers of doom, depending on who you’re talking to. There are two sides to the coin, but unlike the phrase “heads you win, tails you lose,” neither side of the nano coin is necessarily all good or all bad.
The first side of the coin: use in consumer products
It’s certainly true that nanoparticle use in consumer products is increasing quickly. The Woodrow Wilson Center’s Consumer Products Inventory lists over 1200 nanotechnolgy-enabled products. But do the benefits of nanoparticle use in consumer products outweigh the potential harms? With the myriad combinations of particles, coatings, and exposure scenarios, uncertainty remains about the fate, transport and transformation of nanoparticles when released into the environment. All of these factors influence the potential impact on ecosystems and are being studied intensely here at Duke in the Center for Environmental Impacts of Nanotechnology (CEINT).
The other side of the coin: cleaning up pollution
While nanoparticles used in consumer products may be negatively impacting ecosystems, nanotechnology has created significant buzz for its potential remediation capabilities. Many polluted sites (not necessarily polluted by nano-containing consumer products) are being assessed for or are already implementing nanoremediation techniques. By using reactive nanoparticles, scientists are hoping to improve the standard energy intensive, backhoe and bulldozer methods of cleaning a contaminated site. Instead of removing and disposing compromised soils from Superfund sites and then filling the leftover hole with clean soils, we want to see if we can do the treatment on site for less cost and effort.
Just as CEINT is studying the first side of the coin, Duke’s SRC is studying the second side. Project 4 is looking specifically at ways to remediate contamination and the secondary effect those remediation strategies may have on bacteria in either the water or soil. Naturally occurring bacteria that can be found growing in Superfund sites has often adapted to withstand or even sometimes grow off of the chemical contamination. These bacteria might even help us in our degradation efforts.
The figure below shows our approach to the problem. By looking at all three points of the triangle, we’re hoping to engineer ways to degrade contaminants without negatively altering the biology of the site. It’s great to be able to say you’ve removed harmful compounds, but less of a victory if the process of doing so kills everything that was already there! Just like the first side of the coin, using nanotechnology for cleaning up pollution may not be all good – more research is needed.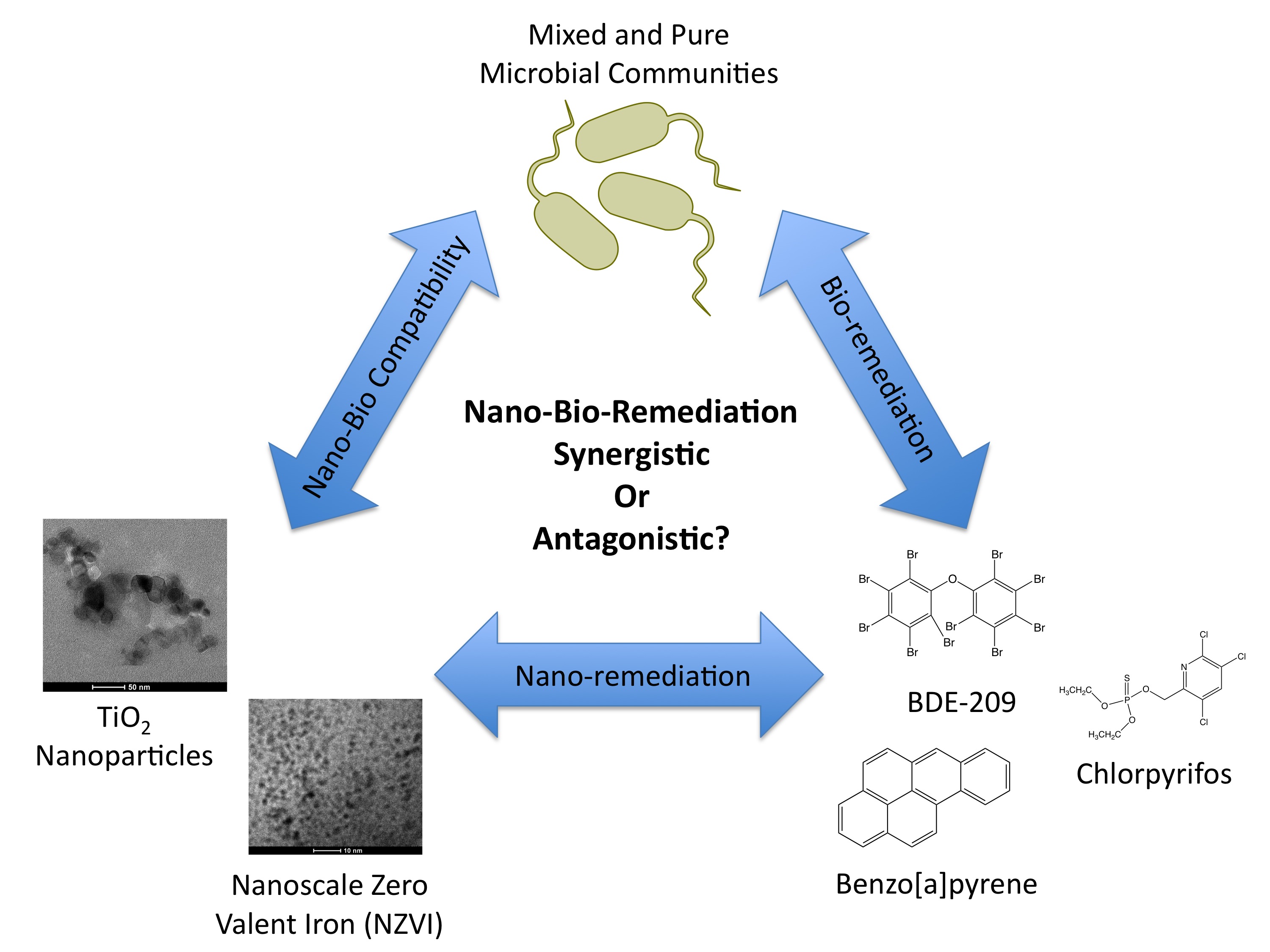
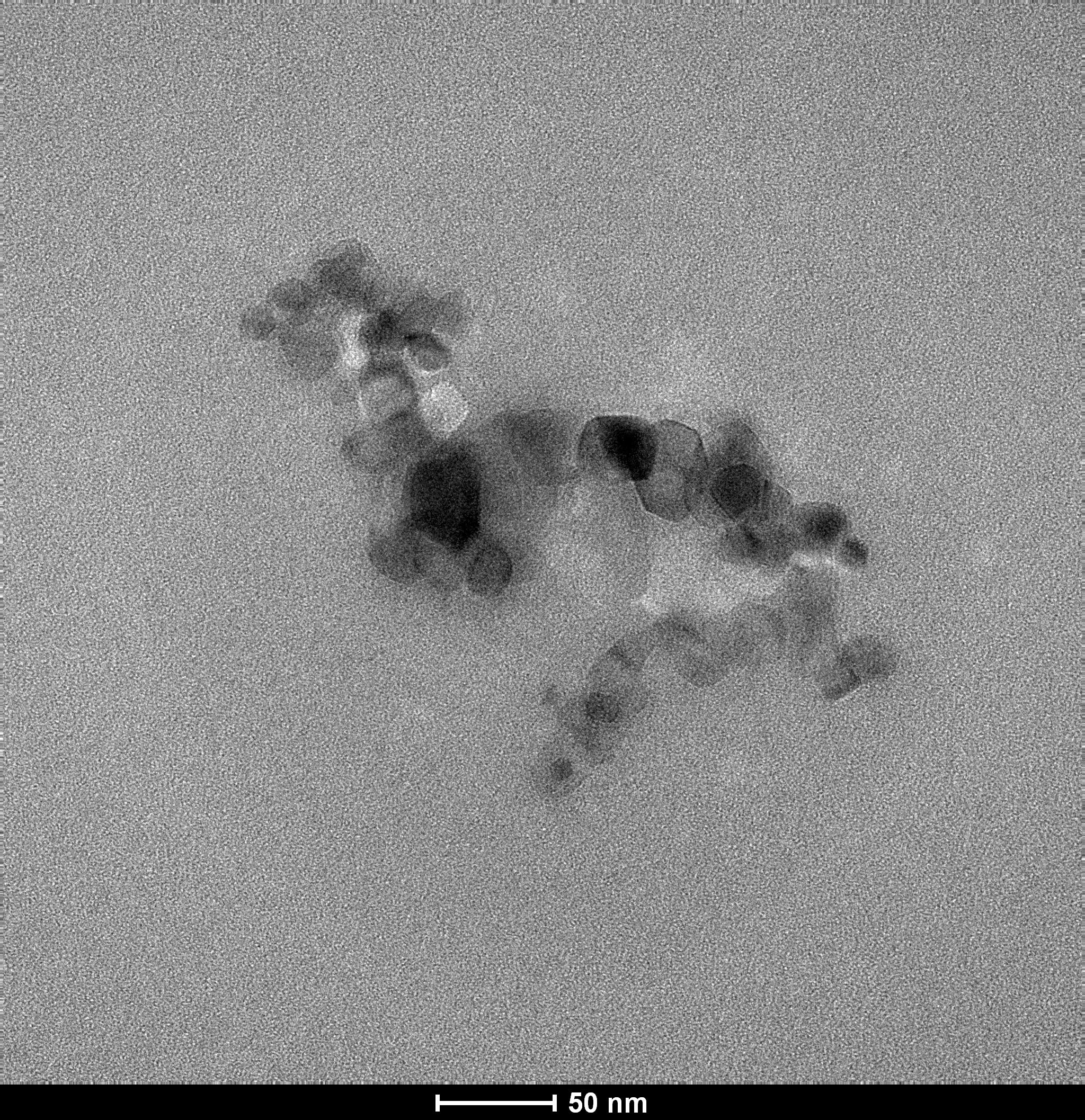
Like Audrey’s work with PAHs, I’m using titanium dioxide (TiO2). By shining UV light on TiO2 nanoparticles that form small aggregates approximately 200nm in size (see image to the right), we’re able to create highly reactive forms of oxygen (called ROS– or “reactive oxygen species”) that break down compounds in water such as the pesticide chlorpyrifos.
My experiments are focused on figuring out how to degrade chlorpyrifos, as well as determine the effect ROS generation has on bacteria in water. The goal is to find a way to clean surface waters that have pesticides in them before they reach larger bodies of water like lakes or rivers. One of the questions that remains is which compounds we’re forming when we degrade chlorpyrifos and whether or not those compounds are more or less harmful for the bacteria in water. As seems to always be the case, one answer leads to even more questions…




